Reverse Osmosis
Reverse Osmosis
Anyone who has been through a high school science class will likely be familiar with the term osmosis. The process was first described by a French Scientist in 1748, who noted that water spontaneously diffused through a pig bladder membrane into alcohol. Over 200 years later, a modification of this process known as reverse osmosis allows people throughout the world to affordably convert undesireable water into water that is virtually free of health or aesthetic contaminants. Reverse osmosis systems can be found providing treated water from the kitchen counter in a private residence to installations used in manned spacecraft.
Reverse Osmosis is a technology that is found virutally anywhere pure water is needed; common uses include:
- Drinking Water
- Humidification
- Ice-Making
- Car Wash Water Reclamation
- Rinse Waters
- Biomedical Applications
- Laboratory Applications
- Photography
- Pharmaceutical Production
- Kidney Dialysis
- Water used in chemcial processes
- Cosmetics
- Animal Feed
- Hatcheries
- Restaurants
- Greenhouses
- Metal Plating Applications
- Wastewater Treatment
- Boiler Water
- Battery Water
- Semiconductor production
- Hemodialysis
- How Reverse Osmosis Works
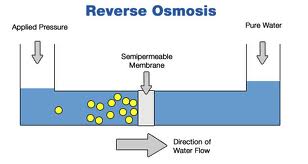
A semipermeable membrane, like the membrane of a cell wall or a bladder, is selective about what it allows to pass through, and what it prevents from passing. These membranes in general pass water very easily because of its small molecular size; but also prevent many other contaminants from passing by trapping them. Water will typically be present on both sides of the membrane, with each side having a different concentration of dissolved minerals. Since the water i the less concentrated solution seeks to dilute the more concentrated solution, water will pass through the membrane from the lower concentration side to the greater concentration side. Eventually, osmotic pressure (seen in the diagram below as the pressure created by the difference in water levels) will counter the diffusion process exactly, and an equilibrium will form.
The process of reverse osmosis forces water with a greater concentration of contaminants (the source water) into a tank containing water with an extremely low concentration of contaminants (the processed water). High water pressure on the source side is used to "reverse" the natural osmotic process, with the semi-permeable membrane still permitting the passage of water while rejecting most of the other contaminants. The specific process through which this occurs is called ion exclusion, in which a concentration of ions at the membrane surface from a barrier that allows other water molecules to pass through while excluding other supstances.
Semipermeable membranes have come a long way from the natural pig bladders used in the earlier osmosis experiments. Before the 1960's, these membranes were too inefficient, expensive, and unreliable for practical applications outside the laboratory. Modern advances in synthetic materials have generally solved these problems, allowing membranes to become highly efficient at rejecting contaminants, and making them tough enough to withstand the greater pressures necessary for efficient operation.
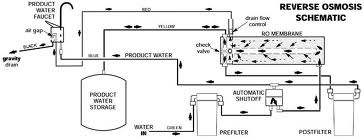
Even with these advances, the "reject" water on the source side of a Reverse Osmosis (RO) system must be periodically flushed in order to keep it from becoming so concentrated that it forms a scale on the membrane itself. RO systems also typically require a carbon prefilter for the reduction of chlorine, which can damage an RO membrane; and a sediment prefilter is always required to ensure that fine suspended materials in the source water do not permanently clog the membrane. Hardness reduction, either through the use of water softening for residential.
 English
English
 Italiano
Italiano Español
Español